Where It Comes From; How to Prevent It; At What Cost
(SUMMARY)
Barry Commoner
Mark Cohen
Paul Woods Bartlett
Alan Dickar
Holger Eisl
Catherine Hill
Joyce Rosenthal
June 1996
CBNS
CENTER FOR THE BIOLOGY OF NATURAL SYSTEMS
QUEENS COLLEGE, CUNY, FLUSHING, NEW YORK
http://www.qc.edu/CBNS/dxnsum.html
This summary report describes the chief results of a two-year CBNS study of the origin of dioxin entering the Great Lakes and the economic feasibility of preventing this process. Full accounts of this work, including sources of data, methodology, the detailed results, and references can be found in two reports:
"Quantitative Estimation of the Entry of Dioxins, Furans and Hexachlorobenzene Into the Great Lakes from Airborne and Waterborne Sources," May 1995
"Zeroing Out Dioxin in the Great Lakes: Within Our Reach," June 1996
These reports and additional copies of this summary are available from:
Center for the Biology of Natural Systems (CBNS)
Queens College, CUNY
Flushing, NY 11367
Tel: 718-670-4180
Fax: 718-670-4189
http://www.qc.edu/CBNS/
II. Dioxin Fallout on the Great Lakes
A. The Two Routes to the Lakes
B. How Much Dioxin Do the Sources Emit Into the Air?
C. Following Dioxin Through the Air
D. The Results: How Much Dioxin Reaches Each of the Great Lakes from Each of the 1329 Sources
E. Conclusion: What We Have Learned About Dioxin Fallout
III. The Feasibility and Cost of Zeroing Out Dioxin
A. The Strategic Approach1. Pollution prevention
2. Dioxin and combustion
3. The economics of dioxin-free production technologies
B. Dioxin-free Alternatives to the Major Sources of Dioxin1. Medical waste incinerators
2. Municipal solid waste incinerators
3. Pulp and paper mills
4. Iron sintering plants
5. Cement kilns that burn hazardous waste
IV. Conclusions: What We Can Do About the Great Lakes' Dioxin Problem -- And The Nation's
Back to CBNS New Reports
Return to CBNS Homepage: http://www.qc.edu/CBNS/
In this age of environmental pollution, one group of chemicals stands out as the most perilous toxic threat to the general population, and yet, thus far peculiarly resistant to effective remedial action: dioxin. The threat is apparent from EPA's September 1994 dioxin reassessment, which concluded that:
These conclusions define what needs to be done to eliminate -- or at least sharply reduce -- the dioxin threat: Dioxin must be traced from each of the thousands of sources, through the atmosphere (where its fate will be affected by wind, temperature, sunlight and precipitation) to its deposition on cattle feed crops (where the crop's growth stage will influence how much dioxin is absorbed) and to the cattle (which will accumulate dioxin in milk and beef). Then, by ranking each source's contribution to cattle's exposure to dioxin, remedial action can be focused on the major ones. This amounts to an ecosystem approach to environmental policy; it tracks dioxin through the successive ecological sectors that comprise the food chain, defining its impact on the people who depend on it.
This approach is very different from current practice, which only considers the dioxin risk from an individual source on the relatively few people near it. For a long time, such risk estimates have assumed that exposure results from inhaling dioxin-contaminated air or, in the case of infants, from eating soil or household dust that contains deposited dioxin. A typical assessment worked out the maximum cancer risk, over a 70-year lifetime, to a person breathing dioxin-contaminated air at a point downwind of an incinerator where the ground-level concentration of dioxin is greatest -- a region no more than a few miles away. Calculated in this way, the cancer risk from a modern trash-burning incinerator is generally at or close to the "acceptable" one per million limit, or perhaps 10-20 times above it. This is a maximum risk; for people living farther away from the incinerator -- as most of them do -- the risk from that source will be much lower.
Recently, for example in the dioxin regulations for hazardous waste incinerators proposed in April 1996, EPA risk assessments have recognized that nearly all of the exposure is through food, but once again, only the risk to the most exposed individual is estimated. This leads EPA to the notion that a farmer, living near an incinerator and eating home-grown beef (milk and dairy products are not mentioned), is at the greatest risk from dioxin incinerator emissions -- not a very helpful guideline for urban residents or indeed for most of the U.S. population.
We now know that these kinds of assessments greatly underestimate the actual cancer risk from incinerator dioxin emissions. The country's incinerators affect not only the nearby people, but, much more powerfully, through their food supply, the entire population. The incinerators' health impact is not created one by one, but collectively, for each one contributes to the widespread dioxin fallout that, channeled through the air, the crops, and the cattle, contaminates everyone's food supply. This is the painful lesson that ecology teaches: the danger of dioxin is vastly greater than the incinerator risk assessments have led us to believe; it threatens the entire population with an unacceptable risk of cancer and grave hazards to fetal development.
An early, outstanding example of the ecosystem approach is the effort to remedy the toxic pollution of the Great Lakes. In recent years, this has focused on the impact of persistent toxic chemicals, including dioxin and dioxin-like compounds (such as certain PCBs), that, entering the lakes, pass through the lake-based food chain and accumulate in fish, wildlife, and people. Since 1909, the environmental future of the Great Lakes has been the responsibility of a pioneering effort in international ecological collaboration. Mandated by a series of U.S.-Canadian treaties, the International Joint Commission (IJC) has evaluated detailed studies of the lakes' ecological status and has proposed ways of improving it.
The IJC has concluded that only the strategy of pollution prevention can end the toxic threat to the Great Lakes. Present efforts to remedy the environmental impact of toxic pollutants -- including the most recently proposed EPA regulations of incinerator emissions -- are almost entirely based on the strategy of control: a device is appended to the source with the aim of recapturing enough of the pollutant to bring the environmental emissions to some presumably acceptable level. The IJC strategy calls for a different approach. Since the goal of prevention is to completely eliminate the pollutant -- which experience shows is unattainable through control devices -- this must be achieved by transforming the process that actually generates the pollutant so that it is not produced to begin with. This can be done, for example, by recycling municipal waste instead of burning it.
There are several basic ways to study a toxic pollutant like dioxin. To gauge its impact on the environment and human health, we need to ask how much of it gets into ecosystems like those that support the Great Lakes or cattle farms. Then, to do something about it, we need to ask where the dioxin comes from, and -- since so many sources are involved -- which ones are the most important to change or shut down, so that their emissions can be reduced to zero and the problem thereby prevented. Finally, we also need to ask how dioxin gets from the places where it is produced -- chiefly incinerators -- to the Great Lakes, cattle farms, or for that matter to anyplace that will suffer from its toxic effects.
In August 1993, CBNS began a two-year project on the Great Lakes, supported by The Joyce Foundation, to answer these questions and an additional one fundamental to the IJC program of preventing dioxin pollution: What will it cost, in jobs and economic impact, to prevent the major sources of dioxin from producing it? To answer these questions we carried out a study directed toward the following goals:
The results of our study are outlined in this summary. They show that once emitted by the numerous sources, dioxin travels in the air over thousands of miles, creating a toxic fallout that settles out everywhere, contaminating not only the Great Lakes, water, fish and wildlife, but the farms where cattle are raised to produce milk, dairy products and beef as well. In a way, our study confirms the EPA source/atmosphere/cattle hypothesis of dioxin exposure -- but extends it to every cattle farm in the United States and Canada -- and confirms the reality of the grave threat that we face from the dioxin that contaminates the food supply. Our study also shows that the major sources of the dioxin deposited in the Great Lakes, in particular the incinerators that burn municipal or medical waste, can be replaced by dioxin-free waste-disposal procedures with little or no loss in economic activity or jobs -- and even with possible gains.
Dioxin can get into the Great Lakes by water as well as by air. The water route brings dioxin into the lakes from pulp and paper mills, sewage treatment plants and chemical plants that pipe their dioxin-contaminated wastewater directly into the lakes or the streams that flow into them. Direct measurements of the dioxin content of the wastewater and the rate at which it flows into the lakes can tell us how much dioxin they receive annually -- the "loading" from each source. Waterborne dioxin can also enter the lakes in leachate from nearby toxic dumps, but the amounts are difficult to measure. Estimation of the waterborne dioxin is particularly difficult in Lake Ontario and Lake Erie. These lakes are burdened by dioxin leaching from nearby toxic dumps and dioxin-laden sediments resulting from earlier, heavily-contaminated waste water from chemical plants and pulp and paper mills. In the other lakes, waterborne dioxin is relatively small, about four to seven times less than the amount that enters from the air.
While the link between the waterborne sources of dioxin and the lakes is direct and relatively simple, this is not true of the airborne sources. There are few actual measurements of the amounts of dioxin emitted by the thousands of airborne sources; yet, from these limited data we must estimate the amounts emitted by each of them. Then, the passage of the dioxin between each source and the lakes -- which determines how much of the emitted dioxin remains to be deposited when it finally reaches the lakes -- is far from simple or direct. The fraction of the emitted dioxin that arrives over the lakes depends on how it moves with the winds; how fast it spreads by diffusion; and how much is destroyed or carried to the ground en route. Yet, however complex, all this must be worked out if we are to understand the origin of the dioxin burden on the Great Lakes -- and eventually of the dioxin that contaminates the country's supplies of milk, dairy products and beef.
Dioxin has been found in the emissions from many processes: incinerators that burn municipal, medical, hazardous chemical waste, or sewage sludge; copper smelters; wood- and coal-burning furnaces; iron sintering plants (which prepare iron ore and steel plant residues for use in blast furnaces); heavy duty diesel vehicles; gasoline-driven cars and trucks. The amounts of dioxin that these different sources produce vary a great deal; but what they all have in common is combustion -- fuel is burned -- and the presence of chlorine in the fuel. Trash-burning incinerators have been studied most, and we now know that they literally synthesize dioxin by chemical reactions as combustion gases cool down in their control devices or exhaust stacks. There, chlorine (released, for example, from the burning of chlorinated plastics) and organic (carbon-containing) molecules that survive combustion combine to produce dioxin.
The effort to estimate how much dioxin falls on the Great Lakes must begin with basic information about the sources -- in particular, their location and how much dioxin they emit per day. Ideally this should be done by continuously measuring the amount of dioxin flowing through the stack, as is done with simpler pollutants such as carbon monoxide. Unfortunately, this ideal is thus far unattainable with dioxin. Instead, a sample of the stack gas must be trapped and sent to a laboratory for an elaborate and expensive (about $1000 per sample) analysis. Very few of the many sources have ever been analyzed for dioxin, and even those are tested only infrequently.
The EPA and other environmental agencies have used the "emission-factor" approach to get around this problem. Measurements are made at a few -- hopefully typical -- trash-burning incinerators, let us say, recording not only the amount of dioxin emitted from the stacks, under standard (again hopefully) operating conditions, but also connecting that amount to the amount of trash burned. An emission factor -- the amount of dioxin emitted per ton of trash burned -- can then be calculated. Finally the amount of dioxin emitted by an untested incinerator can be estimated by multiplying the amount of waste it burns (the "throughput") by the appropriate emission factor.
There are difficulties with this approach, for the amount of dioxin emitted depends, not only on the amount of material burned, but on a number of other factors as well, including: the nature of the fuel (especially its chlorine content); the design of the incinerator; and the type of emission control device. The largest -- and often unknown -- variable is the nature of the fuel. For example, two measurements of dioxin emissions from a Columbus, Ohio municipal waste incinerator (now closed) differed by a factor of five, apparently because of a difference in the composition of the trash burned on the two occasions.
Another, even more basic, difficulty is that the number of certain sources that are actually operating may be unknown. For example, in 1993, when the EPA analysis was made, there was no comprehensive national inventory of medical waste incinerators that listed them by location and throughput. (In contrast, this information was available for trash-burning and hazardous waste incinerators.) In that analysis, and in our own, it was necessary to make a very approximate estimate of the amount of medical waste burned. EPA used permit data from 20 states on individual hospital incinerators to estimate the average amount of medical waste incinerated per capita. This average was applied to other states, based on their population. Since most of the incinerator locations were unknown, for the purpose of our computer analysis the best that could be done was to assign the amount of waste burned to a geographical area such as a state or a large city, based on its population.
Taking such difficulties into account as much as possible, EPA estimated the emission factors of a number of dioxin sources for the 1994 dioxin reassessment. In our Great Lakes study, we brought the EPA data up to date and improved on them somewhat. The range of uncertainty in the emission factors is nevertheless quite large; most of them vary over a 10-fold range or more.
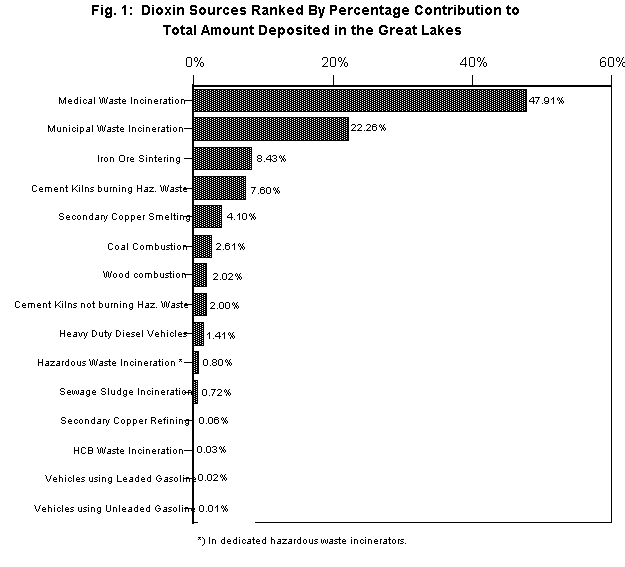
In this way, the emission factors for the various types of sources, their location, and their respective throughput (for example, the number of tons of trash burned by an incinerator, or the amount of fuel used by diesel trucks) were tabulated. We created a database of 1329 sources, of which 954 were individual facilities at specified locations and 375 were aggregated for entire states, provinces or metropolitan areas. We computed the amount of dioxin that each of the various sources emitted into the air from the product of the emission factor and the throughput. Based on average emission factors, this showed that, as of 1993, medical waste incinerators accounted for 53% of the total national dioxin emissions, and municipal waste incinerators for 24%, with about 20% produced by the next five combustion sources (cement kilns burning hazardous waste, secondary copper smelters, wood-burning, iron sintering and coal burning), and the remaining three percent distributed among seven minor sources. Since 1993 this situation has changed considerably; for example, a number of medical waste incinerators have been shut down.
The amount of dioxin that reaches the Great Lakes -- or, for that matter, any other ecological target -- depends on the amount that the sources emit into the air and how much of that is lost (destroyed or deposited) in transit or goes somewhere else. As soon as the dioxin leaves the source, some of it, chiefly attached to relatively large particles, settles out on the ground nearby. Only about 1-10% of the emitted dioxin is deposited within 30 miles of the source. The rest, in the form of small particles and vapor, moves with the wind and spreads over an ever-increasing area. Meanwhile, subject to gravity, the vertical movement of the air, diffusion, and precipitation, some of the dioxin falls to the ground, reducing the amount that is still airborne and able to reach the Great Lakes. At the same time, there are destructive processes at work: sunlight can destroy dioxin depending on whether it is in the form of vapor and therefore exposed to the ultraviolet radiation, or attached to solid particles, and thereby to some degree shielded from it. What is more, whether the dioxin is attached to protective particles or in the form of vapor depends on the temperature; there is a higher proportion of vapor in warm air. Finally, when the dioxin reaches one of the lakes, the amount that comes down depends on the local weather conditions at the time. Rain or snow will carry dioxin quickly to the lake surface.
Fortunately, there is a way to trace dioxin through this complex web. A basic method for tracking certain airborne pollutants was worked out by the National Oceanic and Atmospheric Administration (NOAA) in response to the need to trace the movement of radioactive material -- for example, from a nuclear accident -- in time to warn people who might be exposed to it. NOAA has developed a computer model (called HYSPLIT, after Hybrid Single Particle Lagrangian Integrated Trajectory). It incorporates detailed weather data for the United States, southern Canada and northern Mexico for a grid of 924 points 183 km apart at six levels up to 3,000 meters, recorded at two-hour intervals for every year since 1988. The computer model starts with a "puff," containing a fixed amount of material, emitted at set intervals into the air from a source at a known geographical location. It then tracks each puff as it spreads, moves with the weather, and the material in it is destroyed in transit, or is deposited. Dr. Mark Cohen of the CBNS staff has modified HYSPLIT to incorporate the behavior of the 17 molecular forms of toxic dioxins and furans and eight additional non-toxic groups, in particular with respect to their distribution between the vapor and particulate-bound phases.
The modified HYSPLIT/TRANSCO model is capable of estimating the amount of a specified dioxin congener emitted by a source located at any point in the United States and Canada that is deposited at any other point or area -- in this case, the area of each of the Great Lakes. In a typical computer run, the model tracks the movement of a series of 1251 puffs emitted from the source at regular intervals over a one-year period. It computes the position of each of these puffs and the amount of the dioxin congener it contains, at hourly intervals over the entire year (1993). The model finally estimates the amount of the congener emitted from the source that is deposited over the area of each of the Great Lakes. We can then compute each source's "air transfer coefficient" (ATC) -- that is, the fraction (or percent) of its emitted dioxin that is deposited in each of the lakes.
In order to reduce the computation time needed to estimate the ATC values for each of the 1329 sources and all of the 25 different dioxin congeners, two interpolation procedures were developed and validated. One of these used the ATC values generated for 28 evenly distributed standard source locations to generate interpolated values for all 1329 sources. Another, based on computer runs on four different congeners at each of the standard source locations, generated ATC values for all 28 congeners from the relationship between the ATC values of the four separate congeners and their physical-chemical characteristics.
How well does the computer model do this job? To answer this question we compared actual two-day measurements of the dioxin concentration of ground-level air made by the Ontario Ministry of Environment and Energy at Dorset, Ontario, Canada (in a rural area) at monthly intervals during 1993 with the total concentrations predicted by the computer model at that location arising from the emissions from all 1329 sources. There was a general agreement, with some departures, between the monthly measurements and the model's weekly predictions. But the predicted and measured yearly average values were very close: 3.4x10-15 g of dioxin (TEQ) per cubic meter and 3.3x10-15 g of dioxin (TEQ) per cubic meter respectively. However, this agreement is based on only the single set of measurements suitable for comparison that was available at the time and needs to be confirmed by further comparisons.
The agreement between the predicted and measured deposition of dioxin leads to another important conclusion: It indicates that the amount of dioxin (as TEQ) in the air at Dorset can be accounted for by the emissions from our inventory of sources -- suggesting that no other major sources are involved. For another airborne pollutant, hexachlorobenzene (HCB), that we also studied, the story was quite different. The computer-predicted values for several Canadian monitoring sites were only about one-tenth of the actual measured values. Apart from uncertainty in HCB emissions, this suggests that most of the HCB found at those sites must have come from sources other than those included in our inventory. HCB is much longer-lived in the atmosphere than dioxin and is more likely to be distributed globally, occurring for example in the Arctic, very far from any sources. Apparently, unlike dioxin, most of the HCB deposited in the Great Lakes region is part of the common global pool to which the U.S. and Canadian sources contribute only a part.
Using the computer model, we were able to estimate the amount of dioxin, from each of the 1329 sources, that was deposited in each of the Great Lakes. Analysis of this mass of data yielded some very useful information.
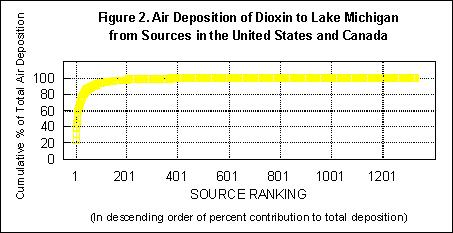
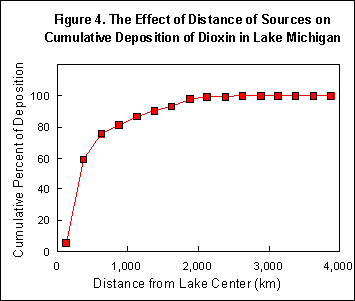
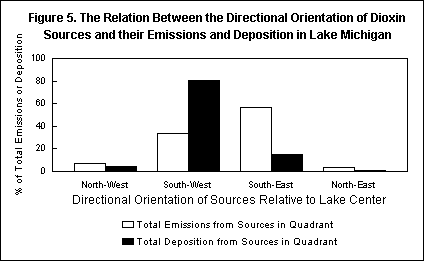
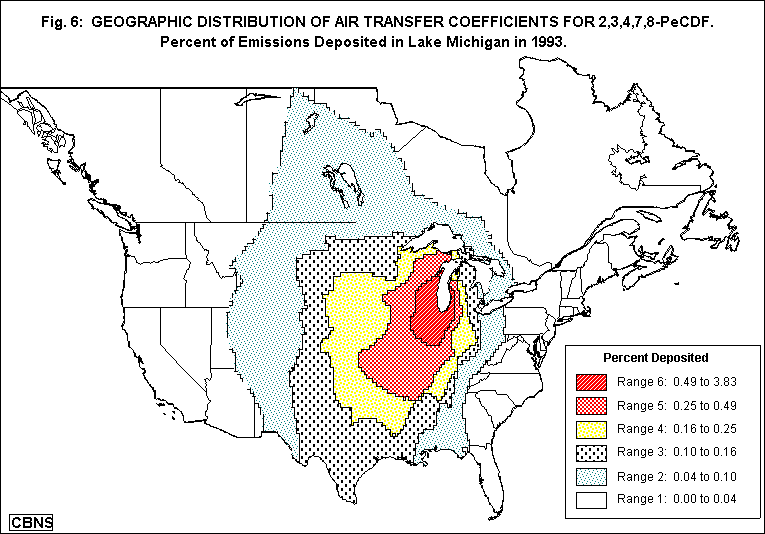
We have learned a number of things that we did not know before, not only about dioxin in the Great Lakes, but also what still needs to be done in order to understand how dioxin contaminates the food supply and what can be done to prevent it. For example, now that we know that a significant amount of the dioxin emitted by trash-burning incinerators and hazardous waste-burning cement kilns as far away as Florida and Texas reaches the Great Lakes, we also know that dioxin fell to the ground everywhere in between -- reaching thousands of dairy farms as well. Thus, we now know that the environmental process that links incinerators to the nation's milk supply is not merely a hypothesis about a local phenomenon, but the path that dioxin actually follows even over long distances. And now that we know from the computer model how to identify, among the thousands of sources, the relatively few that are responsible for depositing most of the dioxin in the Great Lakes, the same could be done for the dairy farms -- leading to plans for protecting them. (A CBNS study of the amounts of dioxin deposited on dairy farms in Wisconsin and Vermont, and the amounts in their feed crops and milk, is underway.)
This new knowledge has important implications for environmental policy. When-- as it is now -- the wisdom of building a trash-burning incinerator is based on the health hazards experienced only in the nearby area, the public policy issues come down to a straight-forward question: Should the community build an incinerator that would expose the people of the community, themselves, to this hazard? Since, in this case, the risk would be self-imposed, the community at risk, through its elected officials, could decide whether or not to accept it.
Now we know that, in fact, the dioxin generated by any one incinerator is combined with dioxin from many other sources, and that their collective impact is visited upon people everywhere -- whether or not they chose to build an incinerator -- through food produced on distant farms. This means, for example, that the risk to the people of Chicago is not so much from inhaling dioxin emitted by the city's trash-burning incinerator, but rather from ingesting milk and cheese produced on farms, for example, in Wisconsin. Moreover, these foods are likely to be contaminated not only by emissions from the Chicago incinerator, but also by emissions from incinerators in Wisconsin, the surrounding states, and states as far away as Florida.
Now the policy debate must be greatly broadened. While the people of Chicago would of course benefit by persuading the city officials to shut the local incinerators in favor of dioxin-free alternatives such as recycling, to really solve the problem the same remedial action would need to be taken in the nearby states, and even in more distant ones. Since the environmental impact of any one incinerator is part of the collective impact of many of them, action to reduce the hazard must be collective as well; the policy issue is no longer local, or even regional, but national.
We have also learned more about the dioxin "background" problem. This goes back to claims made some 20 years ago in reports from the Dow Chemical Company that dioxin occurs throughout the environment because it is created by widespread natural processes such as forest fires, and not by industrial processes and products. That idea was laid to rest when studies of dated lake sediments showed that almost no dioxin was present until the 1940s, and then rose sharply in parallel with the use of chlorine by the petrochemical industry. Nevertheless, in some quarters the idea persists that the widespread occurrence of background levels of dioxin is an unavoidable situation that we have to live with. And it is sometimes argued that since the output of dioxin from any one source does not add significantly to the background level, it can be "safely" operated. We now know that the chief contribution of each source to the hazard of airborne dioxin is, in fact, made by adding to the general level of background dioxin -- the widespread fallout -- and that this dangerous impact on the food supply is not "natural" but man-made.
Another useful outcome of our study has been the identification of serious gaps and inadequacies in the basic information about airborne emissions of dioxin. Only an extremely small fraction of the operating incinerators and other sources have ever been tested for dioxin emissions, forcing reliance on emission factors, which are themselves uncertain. In the case of one major source of dioxin deposition to the Great Lakes -- iron sintering plants -- there are at this time no actual measurements from U.S. or Canadian facilities. In sum, there is a particularly urgent need for more and better information about the sources of airborne dioxin.
In developing a strategy to stop dioxin fallout in the Great Lakes, we have been guided by the principle of pollution prevention. Although pollution prevention has become a popular environmental buzzword in the last few years -- and has even acquired a high-tech shorthand, "P2" -- its practical, operational meaning is often unclear. The clearest way to define pollution prevention is in comparison with its opposite: pollution control. In pollution control, the source that generates the pollutant remains unchanged, but a separate control device is attached to trap or destroy the pollutant before it escapes into the environment. The source continues to produce the pollutant, but now a lesser amount -- which is never zero -- reaches the environment.
Unlike pollution control, prevention calls for changing the technology of the production process in which the pollutant originates, so that the source no longer produces it at all. Automatically, emissions are then zero.
In our first year's work we identified four classes of airborne sources (medical waste incinerators, municipal waste incinerators, cement kilns that burn hazardous waste, and iron sintering plants) that together account for more than 85% of the airborne dioxin deposited in the Great Lakes. We have chosen these sources and one waterborne source (pulp and paper mills) for analysis of economically constructive ways of eliminating their production of dioxin. For each source class, we have aimed to:
All of the sources that emit airborne dioxin are combustion processes, in which a carbon-containing fuel is burned in the presence of oxygen, generating heat. The fuel may be natural gas, oil, coal, or wood (as in a furnace); a mixture of paper, plastic, food scraps, old clothes, and much else (as in a trash-burning incinerator); coke and coal dust (as in an iron sintering plant); or a mixture of toxic organic -- that is, carbon-containing -- chemicals (as in a cement kiln burning hazardous waste). No matter what it is made of, when it is burned, the fuel reacts with oxygen and is chemically changed; its original molecular structure is destroyed. Dioxin, which is, after all, an organic compound, will also burn and thereby be destroyed, if the flame is hot enough. In practice, combustion is never 100% efficient; some fraction -- which may be very small-- of the original organic fuel will survive.
Most of what we know about the relation between combustion and dioxin comes from studies of trash-burning incinerators. When it was first detected in incinerator emissions, it was assumed that the dioxin, having been present in the trash to begin with, had simply survived destruction in the furnace. Incinerator manufacturers claimed that a well-run incinerator would destroy all of the dioxin in the trash and that its presence in emissions was only a sign that the furnace was not hot enough or otherwise malfunctioning. However, a CBNS analysis showed that the amounts of dioxin emitted by different incinerators was not related to their operating temperatures. In 1984, seeking a better explanation, we suggested the possibility that in the cooler parts of the incinerator, surviving fragments of chlorine-free organic compounds in the fuel might combine with chlorine (which is released, for example, when chlorine-containing plastics burn) and produce newly formed dioxin. Within a year this idea was tested in a Canadian incinerator. Dioxin was measured in the fuel (trash), the hot gas leaving the furnace, and the relatively cool gas at the base of the stack. The amount of dioxin at the base of the stack was 100 times the amount leaving the furnace, and much more than the amount in the fuel. We now know that a trash-burning incinerator produces dioxin; whenever the incinerator operates, the world has more dioxin than it had before.
Exactly how dioxin is synthesized in incinerators is not yet known. What is known is that chlorine is essential; if the fuel contained absolutely no chlorine, no matter what else happened in combustion, no dioxin could be formed. Generally, the more chlorine in the fuel, the more dioxin is produced, although other factors may have an influence as well. It also appears that in the form of ordinary salt -- sodium chloride -- chlorine is much less effective in dioxin synthesis than it is when released from an organic substance, such as chlorinated plastic, during combustion. Chloride salts are widely distributed in nature, for example in the oceans, and every living thing. If dioxin could be readily synthesized from chloride, it would be produced in natural processes, for example in forest fires. But this is contradicted by the basic fact that before the 1940s there was little or no dioxin in the environment. The rapid rise in environmental dioxin since then coincides with the increase in the amount of chlorinated organic chemicals produced by the petrochemical industry -- which has thereby created the problem of environmental dioxin.
Since economics is an essential part of environmental policy, it is important to define the targets -- in our case the sources of dioxin -- in economic terms. What these sources -- incinerators that burn municipal or medical waste, cement kilns that burn hazardous waste, iron sintering plants, pulp and paper mills -- do economically is to produce goods or services. They cause environmental problems because the same production process that produces the good or service also produces the pollutant, dioxin, as well. In the pulp mill, the chlorine process produces both bleached pulp and dioxin; in the incinerator, the same furnace that disposes of trash also sets up the chemical reactions that produce dioxin. Pollution prevention is aimed at breaking this link between the economic good and the environmental pollutant, by changing the technology of production so that it produces the good without generating the pollutant -- automatically achieving zero discharge. For example, by recycling instead of burning it, trash is still disposed of, but no dioxin is produced.
Thus, pollution prevention is not a purely "environmental" proposition, but a matter of revising and improving the production process itself. In this sense, except that its motivation is environmental rather than purely economic, pollution prevention is not fundamentally different from a well-established economic process: investing capital to modernize production technologies in order to improve their economic productivity and/or the quality of the products. In the pollution prevention strategy, protecting the environment becomes a decisive goal of investment. And once pollution prevention is seen as an investment in the production process, the importance of evaluating its economic feasibility is self-evident.
For this purpose, we have estimated the changes in the overall cost of producing the sources' goods or services that would occur if the existing dioxin-generating production processes in the Great Lakes region were replaced by dioxin-free processes. For example, we were interested in estimating the change in the cost of operating a regional community's residential waste disposal system that would occur if its trash-burning incinerator is replaced by recycling -- a dioxin-free waste disposal technology.
Waste is generated wherever medical activities occur; about 70% is generated by hospitals; the remaining 30% is due to nursing homes, funeral homes, laboratories, physicians' and dentists' offices, blood banks, veterinarians, and crematories. About 85% of the total medical waste stream consists of the same mixture of discarded paper, plastic, glass, metal and food waste that is found in ordinary household trash. This is the non-regulated, so-called "black bag" waste. The remaining 15% is "red bag" waste, defined as infectious or pathological (surgically removed tissue). Under Federal and state regulations, these wastes must be sterilized before disposal.
In many hospitals both black bag and red bag waste are burned in the same incinerator. Because of the chlorinated plastic now prevalent in medical waste, these incinerators tend to emit more dioxin than ordinary trash-burning incinerators. There are feasible dioxin-free alternatives to the disposal of black bag waste and infectious waste: black bag waste can be either recycled or landfilled, and infectious waste can be sterilized without being burned and then landfilled. Pathological waste (about 2% of the red bag waste or 0.3% of the total medical waste stream) can only be incinerated, in part for cultural or aesthetic reasons but also because it is difficult to sterilize in any other way. Thus, there are dioxin-free means of disposing of 99.7% of the medical waste stream.
There are several alternative dioxin-free means of disposing of infectious waste:
The EPA has not yet formally established regulations on emissions of dioxin from medical waste incinerators, but some states, led by California, have done so. In February 1995 EPA issued draft regulations and, in response to a court order, promised a final version by April 15, 1995. When that time arrived the Natural Resources Defense Council and the Sierra Club, whose victorious suit had set EPA in motion, agreed to another year-long delay. Meanwhile, the draft documents already tell us how EPA plans to deal with the problem. When they finally arrive, the regulations will be based on the principal of requiring incinerators to install the "Maximum Achievable Control Technology" (MACT). We emphasize the word "control" in this title because, in contrast with the IJC approach (and our own), EPA, at least in this instance, has not adopted the strategy of pollution prevention. Indeed, the MACT regulations fly in the face of EPA's own 1989 "Pollution Prevention Policy Statement" by Lee M. Thomas, then EPA Administrator, which calls for a strategic switch from control to prevention. Apparently, in the EPA hierarchy of environmental acronyms, MACT ranks higher than P2.
When they are established, the MACT standards will require medical waste incinerators to emit no more dioxin (and eight other toxic pollutants) than is now emitted by the 12% best-performing incinerators. According to EPA, only dry scrubber/fabric filter control systems with carbon injection can achieve this level of performance.
1) Hospital incinerators: In the Great Lakes states and the province of Ontario, as of 1995 there were 609 hospital waste incinerators in operation. We have evaluated the economic consequence of replacing them with autoclaves or with disposal to commercial facilities. The evaluations were based on the following assumptions:
Results obtained for all 609 hospitals in the Great Lakes region are summarized in Table II, in which the alternative disposal methods are compared in terms of their annual operating costs, which include the cost of the equipment, annualized over a 20-year period at 10%, and annual operating and maintenance costs. They show that:
| DISPOSAL METHOD |
ANNUAL OPERATING COST (Millions of 1994 dollars) |
|---|---|
|
Existing incinerators (uncontrolled) |
9.8 |
|
Existing incinerators with dry scrubber/fabric filter/carbon injection control system (MACT) | 55.5 |
|
Autoclaves plus small pathological waste incinerator | 23.0 |
| Ship to commercial facility | 28.0 |
2) Commercial Incinerators: There are 14 commercial medical waste incinerators in the Great Lakes region. Although their operating costs are not public, they can be estimated from average values developed by the 1993 EPA survey, providing that information about the amount of waste that the incinerator burns ("throughput") is available. This information is available for the commercial medical waste facilities in Ohio which operate four incinerators and four autoclaves. Comparison of the annual operating costs of incinerators and autoclaves, of equal capacity, at the commercial medical waste facilities in Ohio (see Table II) shows that:
| DISPOSAL METHOD |
ANNUAL OPERATING COST (Millions of 1994 dollars) |
|---|---|
|
Incinerators with current control equipment | 2.6 |
|
Incinerators with MACT control equipment added |
3.4 |
| Autoclaves | 2.3 |
|
Autoclaves, plus cost of retiring incinerator debt |
2.9 |
3) Crematories: Like other incinerators, crematories emit dioxin into the air. Their dioxin emissions account for less than 1% of the total emissions due to medical waste incineration. Responding to claims that installing the proposed MACT controls would cost three to seven times the net worth of a typical crematory, EPA has delayed imposing emission regulations on crematories until November 2000.
In response to the proposed EPA regulations on dioxin emissions from medical waste incinerators, existing on-site facilities such as hospital incinerators will be obliged to install the required MACT control equipment, to substitute dioxin-free autoclaves, or to ship their red bag waste to a commercial facility. Commercial facilities will need to install the MACT equipment or substitute autoclaves for their present incinerators. The adoption of the required control equipment would reduce, but not eliminate, incinerator dioxin emissions. In contrast, the universal substitution of autoclaves for incinerators, except for the very small emissions from pathological waste incinerators and crematories, would virtually eliminate the dioxin generated by medical waste disposal.
For hospitals, the least costly alternative is to replace the incinerator with autoclave sterilization of infectious waste (with incineration of the much smaller amount of pathological waste, which is unavoidable). The cost of this replacement would involve less than 0.1% of the overall cost of operating the hospital. Thus, there is no economic obstacle to simply replacing hospital incinerators with on-site autoclaves, thereby eliminating the incineration of all hospital-generated infectious waste. Indeed, the fact that many hospital incinerators have been replaced with autoclaves in the last few years demonstrates the validity of this conclusion in practice.
For commercial facilities, installing an autoclave is less costly than a new incinerator equipped with MACT control equipment. If an existing incinerator is simply replaced with an autoclave, the operating cost of the autoclave is slightly less than that of the MACT-equipped incinerator. However, if the debt on the incinerator's original capital cost is added to the cost of an autoclave, the overall annual operating cost increases by 12% over the existing cost. This cost of the transition to dioxin-free operations could readily be borne by the firm operating the facility. In the case of Browning Ferris Industries, which operates two medical waste incinerators in Ohio, this cost would amount to about one-thousandth of its 1994 revenue.
In sum, there is no significant economic barrier to closing down all medical waste incinerators in the Great Lakes region (except for pathological waste incinerators and crematories). Such a program should begin with the implementation of a waste segregation policy that separately collects black bag waste for appropriate disposal, preferably by intensive recycling, in which all of the recyclable components are recovered. With the hospitals' waste stream thereby segregated into the components unique to medical operations -- infectious waste and pathological waste -- a step-wise transition from dioxin-generating incineration to dioxin-free autoclaving becomes feasible:
The disposal of municipal solid waste (MSW) is, of course, an essential municipal service. It is now met in several ways: landfills, incinerators, recycling, and, to some degree, waste reduction. Landfills might be considered dioxin-free, but only in the sense that they probably do not involve the synthesis of dioxin. However, landfills may contain dioxin-contaminated materials and are, in any case, otherwise environmentally undesirable. Waste reduction is a highly desirable way to improve environmental quality and conserve resources; however, it is not a practical alternative that is capable of totally eliminating incineration and, hence, its dioxin emissions. Recycling is dioxin-free and in many other ways exemplifies sound environmental policy. It is therefore the alternative of choice.
Recycling is a well-established technology of waste disposal that is in fact now more widely used than incineration. Its technological feasibility as a means of constructively disposing of nearly all of the municipal waste stream has been demonstrated in practice. A CBNS pilot test of a system of intensive recycling, which is designed to collect all of the recyclable materials, converted 84.4% of the residential waste stream into marketable paper, metal and glass containers and compost (made from yard and food waste). In such a system, household separation generally segregates the waste stream into four categories for curbside collection: paper; metal, glass and plastic; food (and yard) waste; non-recyclables. The first two recyclable categories are then further processed into marketable commodities (e.g., old newspaper, color-sorted crushed glass, aluminum cans) at a materials recovery facility (MRF). The household-separated organic material (food and yard waste) is converted at a composting facility into a useful soil amendment, compost.
The 1990 Clean Air Act Amendments directed EPA to establish emission limits for MSW incinerators, covering a number of pollutants, including dioxin. The revised regulations were proposed by EPA in September 1994 and promulgated on December 19, 1995. The emission limits are based on maximum achievable control technology (MACT). For existing incinerators, this is defined as the best emission level achieved by 12% of the operating units in a size category. For new incinerators it is defined as the best single unit in a category. Although the 1990 Amendments call for the determination of the residual risks of the emissions after application of the MACT control technology, this has not yet been done.
In 1993, the communities in the Great Lakes region burned 11.8 million tons of residential MSW in 54 incinerators. Based on 1994 and 1995 data, 50 of these facilities are still operating and currently burn 11.7 million tons of trash (two are currently closed but could reopen). We have estimated the economic consequences of closing all the Great Lakes incinerators and creating throughout the region programs of intensive recycling capable of diverting from them at least the tonnage of waste they now burn.
a) Increased collection costs: CBNS studies in New York City have examined the impact of intensive recycling on the recycling rate (that is, the percent of the total waste stream that is separated by households into the several categories of recyclable materials and set out for collection), and the relation between recycling rate and the cost of curbside collection. Based on this study, the introduction of the intensive recycling programs in place of the existing, partial, recycling programs in the Great Lakes region would increase the average recycling rate in the Great Lakes region from 24.7% (in 1994) to 41.9%. This increase in recycling rate would result in a 4% increase in overall MSW collection costs.
b) Increased education costs: Based on our experience in New York City, the cost of public education and outreach programs to acquaint the region's residents with intensive recycling household separation procedures and to encourage participation amounts to about $1 per person per year.
c) Cost and revenue of processing the additional recyclable materials: The net economic outcome of processing recyclables at the MRF depends on the processing costs and the revenue generated by the sale of the recycled materials. In some municipalities, this processing is done by publicly owned MRFs; in others, they are privately owned. Where the MRF is privately owned, the community pays a tip fee and generally receives a share of the revenue from sale of the processed material, which in recent years has been a good deal greater than the tip fee.
We have estimated the net income from processing and marketing the collected recyclable materials, from the average market prices of the various materials in 1994 and 1995, as reported in Recycling Times. Where the MRF is publicly owned, the cost of processing has been estimated from data provided by a major builder and operator of MRFs. These data indicate that a typical 600 tons per day MRF can process the recyclables at a cost of $46 per ton. In recent years the revenue from marketing the processed material has been considerably greater than this cost, so that MRFs have generated net revenue. The cost of processing organic waste into compost is based on data provided by an experienced firm engaged in operating a number of community compost facilities. Based on these data, compost facilities that accept organic waste would charge the community an average fee of $31 per ton for this service in order to operate at a profit. This cost to the community makes good economic sense, however, since it is less than the tip fee charged by incinerators.
d) Avoided disposal costs due to increased recycling: If the 11.7 tons of MSW currently incinerated in the region are diverted to intensive recycling programs, the region's communities would avoid the payment of tip fees to the incinerators. The average tip fee in the Great Lakes region in 1995 was $59.91 per ton in private incinerators and $52.56 in publicly owned incinerators. The weighted average tip fee for the region's incinerators is $57.58 per ton.
e) Cost of incinerator debt retirement: Where the incinerator is community-owned, the community has a financial liability in the form of the unredeemed bonds used to finance its construction. The cost to the community of shifting from incineration to recycling must therefore include not only the cost of the intensive recycling programs, but the cost of paying off the remaining incinerator bond obligation as well. We have therefore estimated the cost of retiring the outstanding bonds on the 52 incinerators in the Great Lakes region, amortized over a 10-year period, from the incinerators' total capital costs, their age, and the nature of the bonds.
2) The net economic impact of substituting intensive recycling for incineration: Given that participation in a recycling program increases after its initial establishment, we have computed the above costs and revenues for the second year after the intensive recycling program is introduced. The annual cost of this change in the technology of MSW disposal is the net sum of the preceding costs and revenues:
The net economic effect of substituting intensive recycling for the incineration of 11.7 million tons per year is summarized in Table III. The increased annual costs of MSW disposal in the region (for additional collection, additional public education, and retiring the outstanding incinerator debt) amounts to $601 million per year. This is outweighed by the additional revenue, $1,137 million, from the marketing of the additional recycled materials and the avoided incinerator tipping fees. In sum, the shift from incineration to intensive recycling generates a net additional revenue of $536 million per year for the Great Lakes communities.
The net revenue from substituting intensive recycling for incineration is, of course, influenced by the variable market price of the recycled materials.
3) Other economic impacts of implementing the intensive recycling program: Beyond the direct economic effect of shifting from incineration to intensive recycling, several other changes in the regional economy will occur:
| COST/REVENUE CATEGORY |
COST IMPACT OF CHANGE IN FIRST YEAR OF INTENSIVE RECYCLING SYSTEM ($millions) |
|---|---|
| Net revenues from additional recycled materials* |
+462 |
| Avoided incinerator tip fees | +675 |
| Additional collection costs | -206 |
| Additional education costs | -88 |
| Incinerator debt retirement costs | -307 |
| Total Net Revenue | +536 |
| *The midpoints of the estimates for public and private facilities are averaged to generate this value. | |
The substitution of intensive recycling for the incineration of residential waste in the Great Lakes region would reduce the emissions generated by this major source of dioxin to zero. This transformation is not only economically feasible but would generate a net savings of about $530 million annually in community waste disposal costs. There would be a net increase of about 24,600 in regional employment.
The Great Lakes region is well situated to accomplish this feat. The U.S. portion of the region already has the highest recycling rate of any region in the United States, and Ontario already recovers more recyclables than any other province in Canada. The region has an excellent recycling infrastructure. Minnesota's Office of Environmental Assistance, Ontario Multi-Material Recycling Inc., and the Recycling Council of Ontario provide municipalities, businesses and environmental organizations with up-to-date information on recycling. The newly established recyclables market at the Chicago Board of Trade creates a national center for recycling commerce in the region.
Because the Great Lakes region has been hard hit by the loss of manufacturing jobs over the past decades, the opportunity for new manufacturing enterprises based on recycled materials would be particularly welcome. With an assured long-term supply of high-quality recyclables, and with the continuing increase in the recycling rate that will occur as the programs mature, the outlook for substantial recycling-based economic development is very good. Because centers like Chicago, Detroit and Toronto generate large amounts of recyclable materials, they are ideal locations for new and/or expanded manufacturing enterprises based on recycled materials.
The following policies and principles of implementation would facilitate this transition:
Bleaching is a major component of pulp and paper production. Until recently, bleaching technology has been based on elemental chlorine gas. This is an effective bleaching agent, but it is equally effective in chlorinating organic compounds, which are released in the pulp-making process. As a result, the wastewater effluent from such operations invariably contains dioxin, as well as other toxic chlorinated organic compounds, often at levels considerably above regulatory standards.
In recent years the mills have increasingly replaced some or all of the elemental chlorine with chlorine dioxide, which is also an effective bleaching agent. In comparison with elemental chlorine, chlorine dioxide forms much less dioxin and other chlorinated organic compounds; but, despite industry claims, the amount formed is not zero. Pulp and paper manufactured in this way is called "elemental chlorine-free" (ECF). Totally chlorine-free (TCF) pulp and paper products, which are produced dioxin-free, can be achieved by using non-chlorine bleaching agents such as hydrogen peroxide.
1) Virgin pulp production: The pulp and paper mills that discharge their effluent into the Great Lakes or their tributaries include ten mills that produce virgin pulp and paper from wood, four of them in Canada and six in the United States. Most of these mills use the kraft process, in which wood chips are heated (in industry language, "cooked") in an alkali solution that breaks down most of the lignin and releases the cellulose fibers that are used to make paper. At this stage, the "brownstock" pulp contains some residual lignin; it is brownish in color and likely to degrade in time. After the breakdown products are washed out of the pulp, it is treated chemically -- with chlorine, for example -- to bleach the pulp and break down the remaining lignin, enabling the production of bright durable paper. As the industry has developed, these basic steps have been refined and additional sequences introduced, many of them designed to reduce pollution.
Efforts to reduce the amounts of chlorinated organic pollutants that accompany virgin pulp production have led to major changes in the basic technology. When an EPA study in 1987 confirmed that pulp mill effluents contained enough dioxin to seriously affect the edibility of fish, and several environmental organizations sued for remedial action, the industry adopted what it claimed was "an ambitious strategy to virtually eliminate dioxin." Since then, pulp mills have reduced the formation of chlorinated organic pollutants in three ways: limiting dioxin-contaminated inputs; reducing the amount of elemental chlorine used; and/or reducing the amount of organic breakdown products -- especially of lignin -- that remain in the pulp when the chlorine is added. As a result, the dioxin content of pulp and paper mill effluents declined by 60-80% nationally in the United States, according to EPA. A similar decline has probably occurred in the Great Lakes.
The industry's goal of the "virtual elimination" of dioxin is based on the view that chlorine dioxide, unlike chlorine, will not significantly chlorinate organic residues. However, the substitution of 100% chlorine dioxide for chlorine in ECF pulp-making does not, in fact, entirely prevent the formation of chlorinated compounds (classified as "AOX"), which are only reduced by about 80%. Some dioxin is produced as well. When chlorine dioxide is added to the pulp, chemical reactions generate a small amount of elemental chlorine -- which in turn reacts to produce a correspondingly small amount of dioxin. Thus, to entirely prevent the production of dioxin, both elemental chlorine and chlorine dioxide must be eliminated from pulp production; only TCF pulp is produced dioxin-free. A recent, highly sensitive comparison of the dioxin content of samples of ECF and TCF pulp produced by the same mill showed that while a measurable amount of dioxin (in the form of tetrachlorinated furan) was formed in the ECF bleaching process, there was no evidence of dioxin formation in the TCF process.
There is, however, a realistic limit to what can be accomplished even though, as in a TCF system, no chlorine in any form is added to the pulp-making process. As our earlier work showed, airborne dioxin now occurs universally in the United States and Canada. This means that pulp mill operations -- and even the trees that are made into pulp -- are contaminated, if only slightly, by dioxin fallout. As long as the numerous sources continue to inject dioxin into the air, there is no way, anywhere, to avoid exposure -- short of operating in an enclosed, purified atmosphere.
2) Recycled pulp production: There are 10 Great Lakes mills that produce recycled pulp. Since they manufacture pulp from paper rather than wood, the basic pulping process is relatively simple (water and agitation are used to mash the waste paper into pulp), but is complicated by the ink and other impurities associated with most waste paper. Reagents release the ink from the paper fiber; the ink particles are then separated from the pulp, for example by flotation, and collected as a waste sludge. Many grades of waste paper contain much less lignin than virgin brownstock pulp, and the recycled pulp can be bleached to a satisfactory brightness with much less bleaching agent. Nine of the 10 Great Lakes deinking plants presently use chlorine or hypochlorite (which also chlorinates organic compounds) as bleaching agents. According to EPA, this does not produce "significant" amounts of dioxin, presumably because the recycled pulp contains only small amounts of organic compounds that, reacting with chlorine, might form dioxin. In any case, recycled paper can be produced with non-chlorine bleaching agents such as hydrogen peroxide or sodium hydrosulfite, yielding "process chlorine-free" (PCF) pulp. However, even such pulp will contain some dioxin if the waste paper from which it was made was chlorine-bleached and therefore likely to contain dioxin.
In 1993, under a consent decree resulting from a suit brought by environmental organizations, EPA issued court-ordered proposed regulations on dioxin in pulp mill effluents. The limits were based on "Best Available Technology," which EPA defined as complete (100%) substitution of chlorine dioxide for elemental chlorine and the use of oxygen or extended cooking to improve delignification. The final regulations are expected sometime in 1996, with implementation in 1999. The industry has responded to the proposed regulation with investments in chlorine dioxide equipment, in effect adopting the ECF approach. However, industry associations have lobbied against the improved delignification requirements, which have nevertheless already been adopted by some companies, several of them in the Great Lakes region.
The nine Great Lakes kraft mills are at various stages in the process changes that have been made to reduce the dioxin content of their effluents. As of 1996 four of them -- Avenor, James River-Marathon, Champion International, and Potlach -- had adopted 100% chlorine dioxide bleaching, and two, E.B. Eddy and Kimberly-Clark, are equipped to do so on a temporary basis. Three mills regularly use a mixture of chlorine and chlorine dioxide; two mills still use 100% elemental chlorine and hypochlorite.
We have evaluated the economic consequences of converting these existing plants into each of four alternative processes: ECF-1 (100% chlorine dioxide, introduced immediately after brownstock washing); ECF-2 (100% chlorine dioxide bleach preceded by oxygen delignification); ECF-3 (100% chlorine dioxide bleach preceded by oxygen and ozone delignification); and TCF (hydrogen peroxide bleach, preceded by oxygen and ozone delignification). The three ECF processes produce progressively less AOX and, presumably, dioxin since the oxygen and ozone treatment reduces the pulp's lignin content, and less chlorine dioxide is needed to bleach the remaining lignin. The TCF process produces no dioxin at all. In each case, we have estimated the change in the cost of the plant's present pulp production process that would occur if it were converted to the new design.
For this purpose we have applied data developed by two recent (1995) studies about the additional equipment and operating costs needed to convert existing kraft pulp mills to ECF-1, ECF-2, ECF-3, or TCF operations. The two studies differ in their approach to capital equipment costs. The Radian Corporation study included only the minimum equipment needed to make the conversion; the study by the Environmental Defense Fund's Paper Task Force included, as well, additional equipment modernization that the mills would likely make to take advantage of the necessary plant "downtime." Actual conversion costs are likely to fall between these two estimates. By adapting the data provided by these studies to the characteristics of each of the nine Great Lakes kraft mills, we have estimated the impact of each of the four conversions on the per-ton cost of producing pulp. The results, expressed as the average (weighted according to each mill's output) for all nine Great Lakes plants, are shown in Table IV.
| Process | Capital, Millions US$ (Aggregated) |
| |||
|---|---|---|---|---|---|
| Total | Annual | Capital | Operations & Maintenance | Total | |
| Radian Costs Applied (Minimum Capital Expenditures) | |||||
| ECF-1: Chlorine dioxide | -- | -- | -- | -- | -- |
| ECF-2: Oxygen delignification; chlorine dioxide | 150 | 20 | +6 | -3 | +4 |
| ECF-3: Oxygen delignification; ozone; chlorine dioxide | 225 | 30 | +9 | -3 | +6 |
| TCF: Oxygen; ozone | 225 | 30 | +9 | +7 | +17 |
| Paper Task Force Costs Applied (high capital expenditures, includes ancillary costs) | |||||
| ECF-1: Chlorine dioxide | 160 | 21 | +11 | +7 | +19 |
| ECF-2: Modern oxygen delignification; chlorine dioxide | 285 | 38 | +16 | -0 | +16 |
| ECF-3: Advanced low effluent; oxygen delignification; ozone; chlorine dioxide | 440 | 58 | +18 | +2 | +20 |
| TCF: Advanced low effluent; oxygen delignification; ozone | 450 | 60 | +19 | +1 | +20 |
| (Note: Numbers do not always add, due to rounding) | |||||
The main results, based on the Paper Task Force analysis, are:
The fact that the increased costs of producing ECF-3 and TCF pulp are essentially equal has important policy implications. The proposed EPA dioxin regulations are based on the use of chlorine dioxide, the approach also favored by industry. Yet, since TCF can be produced at no greater cost than ECF-3, there is no economic reason not to realize the environmental advantages of TCF: i.e., zero dioxin generation and equipment readily converted to totally effluent free operation. Indeed, recent studies of the cost of newly constructed ("greenfield") kraft mills tend to support this conclusion. One study concluded that a new TCF plant with additional closed-loop (and therefore effluent-free) modifications would produce pulp at a cost $35 per ton below the cost at the ECF plant. A critical review of that study concluded that the two costs would be nearly identical.
It seems evident that to reduce to zero the waterborne dioxin entering the Great Lakes from kraft pulp mills, TCF is the technology of choice.
The substitution of non-chlorine-based bleaching agents for chlorine-based agents in recycled pulp plants involves little or no new equipment. The cost differential between the alternative bleaching strategies is influenced only by the relative cost of the bleaching agents and the cost of the waste paper (in industry language, "furnish"). Sodium hypochlorite costs about half as much as the chlorine-free alternatives, sodium hydrosulfite or hydrogen peroxide. However, in the absence of chlorine, the recycled pulp can be made from inexpensive lignin-containing waste paper (for example, old newspaper) instead of more costly lignin-free furnish (such as office paper), since, unlike hypochlorite, the chlorine-free bleaches do not discolor lignin.
We have assembled data on several alternative ways of producing recycled tissue-grade pulp in order to estimate the relative costs of manufacturing it with sodium hypochlorite bleach or sodium hydrosulfite/hydrogen peroxide bleach at the same brightness. When the process uses only high-priced lignin-free furnish and is chlorine-free (PCF), the cost of production is about $4.60 more per ton than the cost of production with a comparable, chlorine-based process. This extra cost represents only 2% of total material costs and less than 1% of the price of deinked bleached market pulp (in July 1995, $540 per ton). However, when the chlorine-free process makes use of cheaper furnish, such as old newspaper, as a substitute for a third of the lignin-free furnish, then PCF production costs about $20 less per ton than hypochlorite-based production. It is not surprising, therefore, that deinking mills in the Great Lakes states, such as Encore Paper in South Glens Falls, NY, have converted to process chlorine free bleaching (PCF).
It is fair to say that although ECF pulp mills are a considerable environmental improvement over mills that use elemental chlorine, it is only the TCF process that can achieve the goal of entirely eliminating the dioxin that the industry continues to impose on the Great Lakes. But there is an important economic barrier to reaching this goal: adopting TCF would increase the Great Lakes pulp mills' production costs more than the ECF production process specified by the proposed EPA regulations (i.e., ECF-2). This raises the question of whether TCF pulp and paper can command a higher price than ECF products and thereby compensate for the differential in production costs. In turn, the economic feasibility of charging more for TCF paper than for comparable ECF products depends on the demand for TCF. As a practical matter, the Great Lakes pulp and paper companies are not going to invest in TCF equipment unless they can foresee a growing demand for it.
The demand for paper products in the Great Lakes region arises chiefly from publishers and printers of books, magazines, catalogues. Moreover, the regional demand is sufficient to absorb the total supply manufactured by the Great Lakes mills. Consequently, an increased demand for TCF paper on the part of the region's printing and publishing industry might well persuade the Great Lakes pulp and paper mills to invest in the transition to TCF. In fact, we can already see the beginning of such a demand-driven shift. For example, the University of California Press, one of the largest university publishers in the United States, which contracts for its books mostly with printers in the Great Lakes region -- chiefly in Michigan, New York and Pennsylvania -- expects to use TCF paper for half of its new titles by 1997.
Ultimately, the demand for environmentally benign products like TCF paper comes from the environmentally informed public. It is no surprise that a university press and a California publisher, Jossey-Bass, should lead the way, for they expect their readers to welcome TCF books. Government agencies are another important way to channel public demand into action. Several states and cities have passed ordinances to encourage the procurement of TCF paper products, among them Oregon, Massachusetts, Seattle, and Chicago. In 1993, environmental organizations managed to include a preference for TCF in a draft Executive Order on Federal paper procurement, but it was dropped from the final draft.
Thus, a kind of economic but environmentally motivated daisy-chain leads from the public, through environmental organizations, academic book publishers, the printers who supply them -- and finally to the pulp and paper mills in the Great Lakes region. As the pull of increased demand for TCF product grows, economic considerations -- if not environmental concern -- may persuade the mills to respond.
Another tug on the Great Lakes mills toward TCF comes from abroad, where European environmental organizations have campaigned strongly for TCF. In 1990 a German governmental scientific group called for an end to the use of all forms of chlorine in the paper industry, and a year later the government advised (but did not require) the German paper industry to do so. The idea soon spread, so that, led by the paper industry in the Nordic countries, by 1993 TCF kraft pulp accounted for some 30% of the printing and writing paper market in these countries and Germany. The largest circulation weekly in Germany, Der Spiegel, has converted to TCF paper. The international furniture company, IKEA, now prints its catalogue on paper made from Scandinavian TCF kraft pulp. By 1994, 22 European mills were producing TCF bleached kraft pulp. As it happens, Europe is the U.S. paper industry's largest regional export market. If the Great Lakes pulp mills wish to maintain their international competitiveness, they will need to respond to the growing European demand for TCF. These relationships could also translate the environmentally motivated demand for TCF into economically motivated production of TCF in the Great Lakes mills.
The response of industry and the regulatory agencies to the problem of waterborne dioxin created by pulp mills contrasts sharply with their response to the airborne sources that we have analyzed. The remedial approach to the airborne sources has relied on tacked-on control devices. In contrast, in the last decade the pulp and paper industry, faced with the issue of dioxin pollution, has made changes in the production process itself, applying the strategy of pollution prevention rather than control. And, like the most familiar successes of pollution prevention -- the more than 95% reduction in airborne lead emissions largely achieved by removing lead from the production of gasoline -- the strategy has worked equally well to reduce the dioxin content of pulp mill effluents. To this extent, the recent effort of the industry to deal with the dioxin problem can be regarded as a salutary example to many other industries -- where pollution prevention is more of a slogan than a principle of action.
Yet, the failure of the pulp and paper industry to adopt TCF as the goal of its remedial efforts reveals some unresolved difficulties in the practical application of the prevention strategy. On its surface, the difference between the industry's preference, ECF, and TCF appears to be trivial: Why should the industry aim for zero dioxin (TCF) when the ECF approach has already yielded a 60-80% reduction and promises more? Why make an even more strenuous effort for the last few percent? The reluctance of both the industry and EPA to do so may actually reflect an attitude engendered by their experience with the control strategy. Because of economic as well as thermodynamic constraints, no control system can be perfect, encouraging the view that the practical goal of environmental remediation is to achieve a 90% or more impact, but never zero. When it turned out that replacing chlorine bleach with chlorine dioxide seemed to approach this goal, the industry was satisfied. In a sense, this was a legitimate effort at pollution prevention that accomplished, not the aim of that strategy -- zero pollution -- but the aim of the strategy of control.
Since some dioxin, and even more AOX (20% of the amount in chlorine bleaching) remains in the ECF effluent, the basic economic fault of the control strategy comes into play -- that as long as any pollutant is released, the environmental benefit will be gradually eroded with time as the economy grows and production increases. This creates a contentious, and unnecessary, conflict between environmental quality and economic progress.
In this sense, the ECF approach suffers from a short-term outlook, which affects the industry's potential for eliminating not only dioxin but all harmful effluents as well. This can be accomplished by creating closed-loop systems to remove pollutants from waste water so that it can be reused indefinitely. The agents used in the TCF process yield byproducts that are much more benign (i.e., less likely to corrode piping and equipment) than those produced by chlorine dioxide. TCF is therefore much more amenable to closed-loop designs, and once installed facilitates conversion to a production system that is not only totally chlorine free, but totally effluent free (TEF) as well.
In both the short and long term, TCF is the environmental technology of choice.
Iron sintering is one of the component processes in the production of steel by blast furnaces. If the iron ore is in a finely powdered form, it is not usable in a blast furnace, since it tends to compact and block the flow of hot gas that is essential to the process. By burning a mixture of fine ore and powdered coal or coke, sintering converts the pulverized material into a lumpy agglomerate that facilitates gas flow in the furnace. Sintering also can be applied to powdery iron-containing residues from the steel-making process. Blast furnaces and other types of steel-making facilities, and indeed the sintering plants themselves, accumulate iron-rich flue dust which must be removed from time to time. In addition, when steel is processed in a rolling mill, or treated with acid, rust-like surface scale is formed that must be removed -- again yielding iron-rich material suitable for sintering. Thus, sintering is a useful way of recovering the iron content of such process residues which would otherwise be discarded as waste. Fine ore can also be prepared for the blast furnace by compressing it into pellets, a process generally done at the mine. In this case, no sintering is necessary. There are 12 iron sintering plants in the United States and Canada, all but two in the Great Lakes states and Ontario.
No U.S. or Canadian sintering plants have been tested for dioxin emissions. However, tests of German sintering plants show that all of them emit dioxin, which appears to originate in the process residues -- which often contain chlorinated organic compounds -- that are included in the sinter mixture. Accordingly, one dioxin-free alternative to current practice is to dispose of the chlorine-containing process residues by landfilling instead of sintering them. Alternatively, the use of chlorinated organic compounds in steel-making might be eliminated. Although the available data regarding either of these alternatives are not well developed, there is enough information about the cost of landfilling to enable an analysis of its economic feasibility as an alternative to sintering process residues.
We consider several alternative changes in production technology that could end the use of process residues in sintering operations. Three scenarios are possible. In each of them the amount of iron originally fed into the blast furnace is maintained, so that the level of steel production is unchanged.
A. Landfill the process residues and replace their iron content with additional virgin fine ore in the sintering operation.
B. Landfill the process residues and replace their iron content by adding more virgin ore pellets to the blast furnace feedstock; sintering continues on a reduced scale, using only fine ore.
C. Landfill the process residues and add enough ore pellets to the blast furnace feedstock to compensate for the iron content of the residues; shut down the sintering plants.
Since neither the U.S. nor Canadian environmental agencies have recognized iron sintering plants as sources of dioxin, no regulatory action has been proposed or enacted.
Several economic factors enter into the costs of implementing these dioxin-free scenarios:
These costs, computed for each of the alternative scenarios, are shown in Table V. Three scenarios, which retain the original level of steel production, are considered: In Scenario A the process residues are eliminated from the sinter mixture and replaced by fine ore of equivalent iron content; the residues are landfilled. Scenario B is similar, except that the replacement material is pelletized ore. In Scenario C the sintering plant is simply shut down; both the residue and fine ore previously sintered are replaced with pelletized ore of equivalent iron content. Scenario A increases the cost of sintering plant operations by $229 million; there is no change in employment. Scenario B increases the cost of sintering by $271 million; employment is reduced by 396. Scenario C is most costly for the industry and its workers. Costs would increase by $327 million and 1100 jobs would be lost. These increases in production cost represent 0.7%-1.5% of the price of steel.
At the same time, the iron ore mining areas of Minnesota, Michigan and Ontario that supply fine ore and ore pellets to the firms that operate the sinter plants will also be affected; the firms will buy more ore from the mines. In Scenario B, the increased demand for ore would raise mine employment by 413 and increase annual wages paid by $16 million -- about equal to the losses at the sinter plants. In Scenario C, there would be 859 new jobs in the mines and $33 million more in wages -- compensating for about 80% of the losses at the sinter plants. In sum, apart from the impact of higher costs to the steel industry and of the loss of jobs to the steel workers, any of the alternative ways to eliminate dioxin production from sintering plants would have a relatively small impact on the region as a whole.
However, these scenarios do not respond to the basic issue raised by the strategy of pollution prevention: where, and in what form, does the chlorine that gives rise to the formation of dioxins enter the steel-making process? Unfortunately, little is known about this basic problem, even in Germany, where most of the studies of dioxin emissions from iron sintering have been done. The most likely candidates are various kinds of chlorinated organic substances that are used in conjunction with steel-making machinery. These include chlorinated solvents, which are common in degreasing and cleaning operations; chlorinated cutting oils, used to lubricate high-speed metal-work machines; extreme-pressure lubricants, such as those used in modern rolling mills; and
| PROCESS CHANGE |
ADDITIONAL COST ($ million) (Relative to cost of existing sintering process) | ||
|---|---|---|---|
| A Replace Residues with Fine Ore |
B Replace Residues with Pellets |
C Close Sintering Plant | |
| Landfill residues | |||
| Replace residues' iron content | |||
| Labor* | |||
| Energy | |||
| Total | |||
| *( ) = change in employment. ** Also includes additional cost of replacing fine ore with pellets of equal iron content. | |||
possibly the fluid used in the hydraulic systems that operate many steel plant machines (the hydraulic fluid may contain chlorinated additives).
From discussions with steel plant engineers, chemical industry representatives and industry consultants, it appears that with one exception, extreme-pressure (EP) lubricants, the chlorinated organic substances now used in steel operations could be replaced with non-chlorinated substitutes.
Unfortunately, there are no publicly available data about the amounts of different chlorinated compounds used in the steel industry as a whole, or the cost of each of their non-chlorine replacements. However, based on data kindly supplied by one of the 25 Great Lakes steel plants (Algoma Steel Inc., Sault Ste. Marie, ON, Canada), we can estimate the total annual consumption of solvents, cutting oils and hydraulic fluid. If these data are extrapolated to the 25 plants that dispose of their process residues to the 12 sintering plants, it would appear that about 26 million liters of these materials are used annually, at a cost of approximately $60 million. If we assume that all of these materials are chlorinated, we can roughly estimate the maximum cost of substitution. Generally, non-chlorinated substitutes are about twice the price of the chlorinated compounds. If it were necessary to replace all of these materials with non-chlorinated substitutes, the cost of making steel would rise by $0.19 per ton, or about 0.04% relative to the price of cold rolled steel. This is, of course, not a definitive result, but only indicates the importance of fully investigating this route to dioxin-free steel-making.
It is likely that certain steel-making operations may have themselves been designed to take advantage of the properties of specialized chlorinated organic compounds. An important case in point is the use of chlorinated lubricants that, because of their non-flammability and effectiveness under high pressure allow operations in which bearing pressures and temperatures are very high (such as hot rolled steel production). Apparently, no existing non-chlorinated lubricant can be used for such machinery. If so, in order to prevent dioxin formation, it would be necessary to exclude residues from extreme pressure operations from the sintering process. The amount of such residues may be significant; the industry uses more than 100 million pounds of extreme pressure lubricants annually.
Our analysis of iron ore sintering operations indicates that there may be ways to eliminate their dioxin emissions without an undue increase in costs by eliminating chlorinated solvents and oils in steel-making. However, so little is yet known about U.S. and Canadian plants that remedial measures and the economic consequences of implementing them remain poorly defined. In these circumstances the most important recommendation that can be made is to use the preliminary information that we have developed as the occasion for establishing a comprehensive survey of the impact of current operating practices in the steel industry on the actual -- that is, measured -- emissions of dioxin from sintering plants. On that basis it would be possible to devise remedial measures for preventing dioxin emissions at the source -- the entry points of chlorine -- and to evaluate their economic feasibility.
As of 1993 there were 28 cement kilns in the United States and two in Canada that burned hazardous waste. Of these, 9 facilities were located in the Great Lakes states and the province of Ontario. They accounted for 7% of the U.S. cement production and 12% of the Canadian production.
Cement kilns are designed to manufacture cement by heating a mixture of raw materials to temperatures in the range of 1400o-1500oC. For that reason, and because of the large amount of material involved, cement production uses a great deal of fuel, generally in the form of natural gas, fuel oil, coal, or coke. The kilns are usually designed to switch fuels easily in response to fluctuating prices. Hazardous chemical waste is massively produced by the petrochemical industry; it is burnable, and cement kilns have been allowed to use it as a substitute fuel. Hazardous waste frequently contains chlorinated organic compounds, including dioxin, and for the reasons discussed above, when it is burned dioxin will appear in the emissions as either surviving or newly synthesized material.
The dioxin-free alternative is quite straightforward: the kiln returns to burning a conventional fuel instead of burning hazardous waste.
On April 19, 1996, EPA issued new proposed regulations governing dioxin emissions from hazardous waste-burning facilities, including cement kilns. They are based on the Maximum Achievable Control Technology (MACT) approach, which sets an emission standard equivalent to the current levels at the 12% best-performing facilities: 0.2 ng (TEQ) per cubic meter. Although, as required by the governing legislation, the April 1996 document includes a section marked "1). Incentives for Waste Minimization and Pollution Prevention," it discusses only waste minimization.
The generation of dioxin by cement kilns that burn hazardous waste can be eliminated by the simple expedient of switching back to their normal fuels: coal, coke, oil, or natural gas. Since the kilns are already equipped to handle these solid, liquid or gaseous fuels, no capital costs are involved in this transition. The transition will, however, affect employment and the cost of operation and maintenance -- in particular, the cost of fuel.
If hazardous waste is replaced with a normal fuel, instead of receiving a tip fee for disposing of the waste (which in 1993 amounted to $68 million), the 9 cement kilns in the Great Lakes region would then pay for the normal fuel (about $9 million per year). This amounts to an increase in their cement production costs of approximately $77 million (see Table VI). At the same time, the transition results in a payroll savings of $11 million, since the additional employees that handle the hazardous material are no longer needed. Finally, if they stopped burning hazardous waste, the kilns could avoid (a) the operation and maintenance cost incident to burning hazardous waste, and (b) the cost of installing the control devices needed to meet the new regulations for dioxin emissions that have just been proposed (April 1996). The cost of burning hazardous waste amounts to roughly $85 per ton of hazardous waste burned, a total of $32 million for the 9 Great Lakes cement kilns burning hazardous waste. According to an EPA-sponsored study, these costs, for improving dioxin emission control equipment in keeping with the proposed regulations, would amount to $19.1 million for the 9 Great Lakes cement kilns.
Table VI: Change in Annual Operating Costs Resulting From Conversion of 9 United States and Canadian Cement Kilns from Burning Hazardous Waste to Burning Coal
| FACTOR | TYPE OF CHANGE | CHANGE IN COST (million 1993 $) |
|---|---|---|
| Energy: coal @ $1.40/MBtu | ||
| Hazardous waste tip fee ($.089/lb) | ||
| Cost of burning hazardous waste ($85/ton) | ||
| Improving current control equipment to MACT standards ($50/ton)* | ||
| Payroll for employees handling hazardous waste ($19/ton) | ||
| Total | ||
| Total conversion impact as % of price of cement: +4% *Based on data from Industrial Economics Inc. (1995) | ||
An important but poorly evaluated economic factor relates to recent changes in the supply of hazardous waste and the capacity to burn it. A recent analysis concludes that commercial incinerators and cement kilns burning hazardous waste are currently operating at only 60-80% of their capacity to burn such wastes. As a result, there is now intense competition for the relatively short supply of hazardous waste among cement kilns and commercial incinerators, which tends to reduce the fees that they can charge. Thus, a recent account of the incinerator industry's objections to the secrecy of current EPA discussions with the cement industry points out that "[T]he controversy comes down to the competition between some cement makers and incinerator operators for a shrinking supply of hazardous waste to burn."
These developments suggest that the added income that cement kilns enjoy by burning hazardous waste instead of normal fuel is likely to be less than our present
estimate indicates. This is especially true because, on top of the over-capacity, the supply of hazardous waste is declining, the result of the environmentally motivated campaign in the chemical industry to reduce the generation of such wastes.
Despite the absence, thus far, of the data needed for a complete analysis of the economic impact of requiring cement kilns to burn normal fuels rather than hazardous waste, it would appear that there will be little or no economic barrier to this transition -- a change that would eliminate this source of the dioxin now entering the Great Lakes. Indeed, the industry itself provides persuasive evidence that it is economically feasible to produce cement without burning hazardous waste. More than three-fourths of the cement is produced, quite successfully, without burning hazardous waste.
We venture, therefore, to recommend that the Great Lakes states and Ontario -- and indeed the U.S. and Canadian regulatory agencies as a whole -- develop regulations that end the practice of burning hazardous waste in cement kilns. As experience shows, this change will not take place in the absence of public pressure. The fact that cement is extensively used in public construction, and that it may be contaminated with dioxin and other toxic pollutants if it includes ash from the combustion of hazardous waste, creates an opportunity to exert such pressure. For example, in 1991 the City of Fort Collins, Colorado, on environmental grounds, passed a resolution against a local cement company's plan to burn hazardous waste in its kiln-- a customary form of complaint. But the Council added a more persuasive argument when it outlawed the use of cement from kilns burning hazardous waste on any City-funded projects.
This report marks the completion of a two-year project to evaluate the technological and economic feasibility of eliminating -- zeroing out -- the major sources of dioxin in the Great Lakes region, where these highly toxic chlorinated compounds have created serious environmental problems. The problems have been well documented in the Great Lakes over the last decade: dioxin and dioxin-like compounds have accumulated in fish; fish-eating bird populations have declined; serious developmental defects have occurred in wildlife. Yet, the most perilous problem is in the general human population. We are exposed to levels of dioxin that create a lifetime cancer risk hundreds of times above the "acceptable" limit and threaten serious defects during fetal development of the endocrine, immune and nervous systems. Our exposure is chiefly through major foods: milk, dairy products, and beef. By any reasonable standard, this means that we must eliminate exposure to dioxin.
The chief outcome of this project is that it provides a guide to remedial action that can reach this urgent goal. The results of the project's first phase showed that, once emitted from the numerous sources that produce it, dioxin travels in the air over thousands of miles, creating a toxic fallout that settles out everywhere -- contaminating not only the water, fish and wildlife in the Great Lakes, but the farms where cattle are raised to produce milk, dairy products, and beef as well. Since there is no way to shield either the Great Lakes or the farms from dioxin fallout, remedial action must be directed at the sources that produce it.
In the second phase of the project, we have developed a remedial strategy designed, not to "control" the entry of dioxin into the environment, but to eliminate its production to begin with. This is the strategy of pollution prevention, so vigorously advocated in the Great Lakes region by the International Joint Commission. This strategy calls for changing the technology of the production process -- which may be the manufacture of bleached pulp at a paper mill, or the production of an essential service, trash disposal, at an incinerator -- in which the pollutant originates, so that the source no longer produces it at all. Pollution prevention does not "control" the emissions of dioxin; it eliminates them.
As we have seen, preventive remedial action is then no longer a purely "environmental" proposition, but a matter of revising and improving the production process itself. In this sense, except that its goal is environmental rather than economic, pollution prevention is not fundamentally different from a well-established industrial process: investing capital to modernize production technologies -- for example, how a paper mill bleaches pulp, or a community disposes of its trash -- in order to improve their economic productivity and/or the quality of the product or service. In the pollution prevention strategy, protecting the environment becomes a decisive motivation for investment. And once pollution prevention is seen as an investment in the production process, the importance of evaluating not only its technological feasibility, but its economic prospects as well, is self-evident.
Based on our first year's work, we identified four airborne dioxin sources (medical waste incinerators, municipal waste incinerators, cement kilns that burn hazardous waste, and iron ore sintering plants) and one waterborne source (pulp and paper mills) for technological and economic analysis. Together they are responsible for about 86% of the dioxin that enters the Great Lakes. For each source class, we have aimed to identify the appropriate changes in production technology that prevent dioxin formation; estimate the cost of substituting them for the existing dioxin-generating technologies; and express this cost relative to the overall cost of production.
We have analyzed the following conversions of the existing dioxin-generating sources into dioxin-free alternatives that are capable of yielding the same product or service:
We have analyzed these conversions on a regional basis by evaluating their effects on those of the five selected source types that are located within the Great Lakes region: the eight adjacent U.S. states, and the province of Ontario in Canada. In the same sense, we have evaluated the resultant economic impact on the overall economy of the Great Lakes region.
The total impact of the conversions on the regional employment and economy is summarized in Table VII. This shows that the transitions can be accomplished with little or no loss in economic activity or jobs -- and even with some gains.
The indicated changes in pulp mills, iron sintering plants and cement kilns burning hazardous waste would reduce employment in the region by about 1,100 jobs. But jobs created in the transition from incinerating trash to recycling it could add some 24,600 to the region's 43 million jobs, for a net gain of more than 23,000 -- a small but positive accomplishment.
Zeroing out the dioxin generated by the region's medical waste incinerators, iron sintering plants, cement kilns burning hazardous waste, and pulp and paper mills would increase their total annual costs by about $370 million. But the approximately $530 million annual savings from replacing trash-burning incinerators with intensive recycling would yield a net savings of about $160 million for the region. Looked at in this rather simple way, the overall impact of the whole program, carried out at once, far from straining the region's resources, would actually help them a bit.
| Dioxin-Generating Source | Employment | Annual Cost | Dioxin-Free Replacement | ||
|---|---|---|---|---|---|
| Number | % of Total Great Lakes | Amount (1994 U.S. $million) | % of Great Lakes Gross Product* | ||
| Municipal solid waste incinerators | (savings) | ||||
| Medical waste incinerators: Hospitals Commercial | negligible negligible | negligible negligible | +21 -3 | +.001 -.0002 | Autoclaves |
| Pulp mills:
Kraft & soda Recycled paper | -80 negligible | -.0002 negligible | +58 negligible | .003 | TCF PCF |
| Iron sintering plants | |||||
| Cement kilns burning hazardous waste | |||||
| Total | (savings) | (savings) | |||
| *The Great Lakes Gross Product is
defined as the sum of the gross products of the eight Great Lakes states and the province of
Ontario. It is $2.034 trillion in 1994 U.S. dollars. Numbers do not always add, due to rounding. Notes: Employment: '94 Employed annual average, U.S.; December '94 Canada. State and Provincial GDP adjusted from 1991 values with national '91-'94 GDP data and price/currency indexes. Sources: The Daily, Statistics Canada, Jan. 5, 1996; Statistical Abstract of the United States, 1995, Dept. Of Commerce, September; OECD, 1996, "Gross Domestic Product," Main Economic Indicators, April; Economic Surveys; United States 1995, OECD; Environmental Performance Reviews: Canada, 1995, OECD; Canada Yearbook 1994, Statistics Canada, 1994; Employment and Earnings, May 1995, U.S. Bureau of Labor Statistics. | |||||
These environmentally motivated changes would have very little impact on the region's overall economy. Again, very simply, we can compare the size of the economic impact with the Great Lakes region's $2 trillion Gross Domestic Product (GDP). The largest positive outcome of the dioxin-free transition, the approximately $530 million per year savings from replacing the trash-burning incinerators with intensive recycling, represents only three-hundredths of a percent of the region's $2 trillion GDP -- hardly a ripple. The increased costs incurred by the overall transition -- which amount to about $370 million annually -- would generate additional economic activity and, demonstrating the perversity of this way of measuring the economy, would actually increase the GDP, but again, proportionally by very little.
In several instances, recent, real-world events confirm our conclusion that the dioxin-free alternatives that we have evaluated are economically feasible. For example, in the last few years numerous hospitals have closed their waste-burning incinerators and have either installed autoclave systems or have consigned their medical waste to commercial facilities, many of which are based on autoclaving. This is consistent with our own finding that autoclaving medical waste is less costly than incineration when -- as required by impending EPA regulations -- improved, more costly emission controls are installed. In the same way, the recent expansion of municipal waste recycling programs, and the decline in the pace of new municipal waste incinerator projects, reflect the recognition by many communities that -- as we have found -- increased recycling can reduce the overall cost of waste disposal. Similarly, particularly in Europe, pulp and paper companies have discovered that totally chlorine-free processes -- which are therefore also dioxin-free -- can be economically successful.
Of course, in the real economic world, nothing is that simple. Our analysis of the cost of replacing medical waste incinerators with dioxin-free autoclaves shows that hospital budgets would rise by about one-tenth of one percent -- a very small proportional increase, but nevertheless money that hospitals find hard to come by. The shift of the regional pulp and paper industry to dioxin-free TCF production might raise the price of pulp by a few percent and put a financial strain on marginal firms. Zeroing out dioxin in the region's iron sintering plants would cause some unemployment in that sector, and might increase the price of steel by one or two percent. On the other hand, because of the change from incineration to intensive recycling in trash disposal, the region's communities could find their perennial budget gaps reduced by a total of about $530 million each year.
Thus, both our analysis and real-world events have demonstrated the practicality of the proposed dioxin-free conversions. In several respects the Great Lakes region is in a good position to undertake them. It is in a particularly favorable position to implement the transition from municipal waste incineration to intensive recycling. The region has the best-developed recycling infrastructure in both the United States and Canada. Three of the region's states -- Pennsylvania, Minnesota and Wisconsin -- have the largest number of recycling programs in the United States; Minnesota has the highest recycling rate of any state in the United States. The region as a whole has more than 3,000 recycling programs in operation. Ontario has a particularly active recycling research and development program, and it is significant that Guelph, Ontario, has established the first city-wide curbside intensive recycling system in North America. In addition, the region is in an excellent position to reap the economic benefits of an increased rate of recycling. It already has a high concentration of deinking mills for recycling paper, and it has highly developed manufacturing facilities that use an array of materials that can be derived from recycling programs.
The Great Lakes region is also well suited to undertake a program that would encourage the paper industry to move rapidly toward totally chlorine-free (TCF) technology. The Great Lakes printing and publishing industry is a major customer of the region's pulp and paper industry, and at the same time is very active in academic publishing -- a sector already interested in using TCF paper. Conditions are therefore favorable for the development of a demand-generated shift toward totally dioxin-free paper production in the region.
What would the Great Lakes gain in environmental quality by eliminating the major regional sources of dioxin? Those sources that are located within the Great Lakes region account for about 57% of the total deposition of airborne dioxin in the lakes -- so that zeroing out their emissions would reduce dioxin deposition by that amount. But the region's decision to undertake this effort would accomplish even more by demonstrating that the dioxin fallout problem could also be solved on a national scale -- thereby eliminating the rest of the major sources' dioxin deposition as well.
First, the same dioxin-free production technologies that, in our analysis, served to replace the dioxin-generating sources in the Great Lakes region will be equally applicable everywhere else. Second, the balance between the economic consequences of these changes and the overall economy is about the same in the Great Lakes region and nationally. The aggregate size of the sources in the Great Lakes (as indicated by their throughput) is about one-third the size of the total U.S. and Canadian sources, and the ratio between the region's GDP and the joint US./Canada GDP is not very different: 27%. So we can expect that a national program to zero out the major sources of dioxin would, like the regional program, have a negligible impact on the U.S. and Canadian economies.
In sum, the transition to dioxin-free replacements for the major dioxin-generating sources could be achieved with very little cost and possibly some gain, not only in the Great Lakes region, but nationally in the United States and Canada. Such national programs would reduce the dioxin fallout deposited in the Great Lakes by about 86% -- a very long step toward the International Joint Commission's goal of virtual elimination. Zeroing out dioxin is within our reach.
We also need to remember that the dioxin generated by these sources is deposited not only in the Great Lakes, but everywhere else as well. The national transition to dioxin-free production technologies would not only protect the Great Lakes ecosystem. It would also shield the crops that feed the cattle on every farm in the United States and Canada from dioxin fallout -- and so end the danger of dioxin-induced cancer and disrupted development that threatens us all.
However, the technological and economic feasibility of these conversions, while a necessary condition, is not a sufficient one. Apart from engineering and economics, a social impulse, provided by a force outside the industry and the agencies that regulate it, is a prerequisite for preventive remedial action. This is evident from the recent efforts to confront the dioxin problem. For example, the rapid pace at which hospitals have chosen to close their on-site incinerators in the last few years is clearly a response to the regulations proposed by U.S. EPA and already enacted by some individual states, such as Ohio. But many of these regulatory actions have not been voluntary; instead, they have been forced by court orders resulting from successful lawsuits by environmentally concerned residents and organizations.
The same process is evident in the recent history of the pulp and paper industry. The industry's trend toward adopting ECF processes clearly reflects EPA's proposal to establish regulations that require such changes -- regulations mandated by the court following two environmental organizations' successful lawsuit. The current campaign of local and national environmental organizations -- and, especially in the Great Lakes, regional ones -- for a transition to totally chlorine-free pulp and paper operations can be seen as a necessary precursor to the industry's movement in that direction.
With respect to municipal waste incinerators, the situation is somewhat different but equally revealing. In the 1980s it was often the EPA and state agencies that encouraged communities to incinerate their waste in preference to disposal in landfills, apparently on the grounds that the pollutants leaching from landfills were a more serious environmental hazard than the emissions from incinerators. At that time, only local community organizations perceived -- correctly, it turned out -- that incinerator emissions, particularly of dioxin, were a serious environmental hazard, a conclusion now confirmed by the 1994 EPA dioxin reassessment. Moreover, well before state and national agencies acknowledged that reuse and recycling of municipal waste is preferable to incineration, local environmental organizations persuaded municipal administrators to establish recycling programs -- often emphasizing that this would enable them to avoid building trash-burning incinerators.
There is an interesting, if ironic, footnote to the history of these relations among the public, regulatory agencies, and industry. For a long time EPA's regulatory approach has been governed by the strategy of pollution control, which -- despite recent proclamations about the importance of pollution prevention -- continues to dominate the Agency's regulatory efforts. However, pollution control is economically unproductive; as standards become more strict, the marginal cost of implementing them rises very rapidly. This gives rise to a dynamic interplay among three forces that influence environmental policy: the public's pressure for improved environmental quality; EPA's tendency to respond -- when it does -- by imposing stricter control-based standards; and the industries' reaction to the resulting increase in environmental costs, which induces them to recognize the economic advantages of pollution prevention over control.
Examples of this process are evident in the sectors that we have analyzed. Thus, in response to public complaint about high levels of dioxin emissions from hospital incinerators, EPA has proposed stricter emission controls; the high cost of these controls has convinced hospital administrators that the prevention strategy -- that is, the installation of a dioxin-free autoclave -- makes economic sense. Similarly, pressured by public protests over dioxin-contaminated fish, regulatory agencies proposed more stringent effluent standards, sharply increasing the pulp and paper companies' potential control costs. In response, the industry adopted the pollution prevention approach and made the less costly process changes that can prevent the generation of dioxin. In this way, public pressure for environmental improvement is translated into a currency more familiar to industry: investment.
Experts -- among them environmentalists, engineers, and economists -- will necessarily contribute what they know to this process. But if the public's concern for the environment is to contribute the driving force, there must be a crucial connection between the experts and the public: the public must learn what the experts know.
While the experts are obliged to inform the debate, they must surrender to the public the right to determine its outcome. There is, of course, no guarantee that the public decision will in fact satisfactorily resolve the problem. But in a democracy this fault cannot be remedied, as recent proposals would have it, by "leaving it to the experts." Environmental policy is better served if it is guided, instead, by the words of Thomas Jefferson:
I know of no safe depository of the ultimate powers of society but the people themselves...and if we think them not enlightened enough to exercise their control with a wholesome discretion, the remedy is not to take it from them, but to inform their discretion.